The History of Genetics page is designed to walk you through the process of the discovery of genes. Along with the date and the scientific discovery that took place on that date, this page also provides a brief background on the scientist(s) who made the discoveries.
 | Charles Robert Darwin was a British scientist who altered our views on the natural world by preparing the foundations of the theory of evolution. In 1858 Darwin proposed the theory of natural-selection; natural selection being the idea that members of a population who are better adapted to the environment survive and pass on their traits, or in other words, survival of the fittest. In 1859 of November, Darwin published what is considered to be the foundation of evolutionary biology: The Origin of Species. |
Gregor Mendel was "an Augustinian monk who was the first person to trace the characteristics of successive generations of a living thing" (Citation 28). In 1866 he presented his results on his experiment on pea plants. He used pea plants to determined the inherited factors that are passed down onto the offspring (Citation 27). In 1956 he began his pea plant experimentation, testing about 28, 000 pea plants (7 pairs of different seeds). Throughout the years, Mendel carefully analysed their growth, as well as their offspring's growth, for comparison. His pea plant experiment eventually led to the discovery of heredity units, or genes. These genes carry characteristics that are either determined to be dominant or recessive; meaning the plant ( or individual) with the dominant gene will show the characteristic in contrast to that carrying the recessive gene. Mendel produced two generalizations that became known as the laws of heredity, which are still used in present day genetics (Citation 29). |  |
 | Johannes Friedrich Miescher was a "Swiss student of cell metabolism and discoverer of nucleic acids" (Citation 31). In 1869 DNA, originally called nuclein, was identified by Friedrich Miescher as an acidic substance found in cell nuclei (Citation 32). The discovery of a substance that contained both phosphorus and nitrogen in the nuclei of white blood cells found in pus by Miescher contributed to the development of knowledge on the concept of genes. At first the substance was named nuclein because it seemed to come from cell nuclei but later became known as nucleic acid during 1874, when Miescher separated it into protein and acid components, now known as deoxyribonucleic acid (DNA). (Citation 31). |
Hugo de Vries began his genetic experiments with plants in 1880 while a professor of Botany at the University of Amsterdam. His hybridization experiments were mostly completed without any knowledge relating to that of Mendel's work and experiments. However, based on his own results, de Vries drew the same conclusions as Mendel (Citation 34). Carl Correns began to experiment with trait inheritance in plants in 1892 while he was a tutor at the University of Tubingen. Originally unaware of Mendel's law of heredity, Correns, however, acknowledged Mendel in his paper he later published by naming it G. Mendel's Law Concerning the Behavior of the Progeny of Racial Hybrids (Citation 35). Erich von Tschermak while studying agriculture at the University of Vienna, worked on a farm to gain practical agricultural experience. He started doing plant breeding experiments in 1898 using peas. He had later written up his results to be published. Like de Vries and Correns, Tschermak i derived "Mendelian" laws of inheritance from his plant experiments independantly (Citation 36). In 1900 de Vries published his work and Mendel's experiments from 1866 were "rediscovered" and confirmed by three separate researchers: (pictured going in order from top to bottom) Hugo de Vries, Carl Correns, and Erich von Tschermak. However, Correns and Vries were the ones who most clearly "redefined" Mendel's laws, with respect to Tschermak who did receive his share of attention for his discovery. They all helped to restate Mendel's results, giving us Mendel's law of segregation and Mendel's law of independent assortment (Citation 35). |  1st picture: (Citation 34), 2nd picture: (Citation 37), 3rd picture: (Citation 36). |
 | Walter Sutton was as American geneticist and physician who presented his theory that the Mendelian laws of inheritance could be applied to chromosomes at the cellular level of living organisms. This theory is now known as the Boveri-Sutton chromosome theory (Citation 39). In 1902 Walter Sutton was pointed out that chromosomes obey Mendel's rules; hence resulting in the chromosomal theory of heredity (Citation 39). Sutton indicated the interrelationships between cytology and Mendelism, eliminating the gap between cell morphology and heredity (Citation 27). |
Sir Archibald Edward Garrod was the son of a physician who diagnosed and studied rheumatoid arthritus. Encouraged by teachers to enter the field of science and medicine as opposed to his father's desire for Garrod to study business, Garrod later went on to be the first to suggest the idea that diseases were "inborn errors of metabolism". He believed that diseases were the result of missing , incomplete, or wrong steps in the body's chemical pathways (Citation 40). In 1902 Garrod linked a human disease to genetic causes. Collecting family history information, as well as urine, from his patients, Garrod was able to determine that alkaptonuria is a recessive disorder. Garrod published a book called The Incidence of Alkaptonuria: A Study in Chemical Individuality. Garrod's book was the first published account of a case of recessive inheritance in humans (Citation 40). |  |
 Reginald Punnet (left) & William Bateson (right) | Reginald Punnet and William Bateson In 1905 Reginald Punnet and William Bateson first reported incomplete linkage in the sweat pea. They formulated the "reduplication" hypothesis that stated that "segregation does not occur at the time of meiosis but somewhat earlier, and not necessarily at the same time for each pair of genes". The offspring of the cells that contain a single set of genes then go on to multiple at random differentiating rates to give the observed ratio (Citation 45). |
  | Nettie Maria Stevens was one of the first female scientists to be acknowledged in biological sciences. Stevens was an intelligent student, always receiving the highest scores among her classmates. Graduating with a masters in biology, Stevens eventually developed a thesis involving a significant amount of microscopic work, along with details of marine life. This training contributed to her success with later investigations of chromosomal behavior (Citation 46). Edmund Beecher Wilson chose to study biology instead of law, although he was the son of a judge. His training consisted of a specification in the field of embryology; considering the fact the genetics did not exist at the time. However, cytology, the study of chromosomes, was becoming more significant, and therefore, Wilson took it upon himself to become an expert in this field. He began investigating the role of heredity in cellular development (Citation 47). In 1905 Nettie and Wilson independently described the behavior of sex chromosomes: XX determines female; XY determines male. Stevens published her work on sex determination as a Carnegie Institute report. In this first study, she looked at sex determination in the common mealworm, discovering that female cells contained 20 chromosomes, but male cells contained 19 large chromosomes and 1 very small one. She showed that the X body paired with a 20th, and significantly smaller, chromosome in meiosis. She suggested that these two chromosomes be called X and Y, explaining that females contained two X chromosomes (Citation 49). Wilson published a paper, Studies on Chromosomes, based on his studies of insect chromosomes. Later that year Wilson was asked to look at Stevens' paper on the subject. Wilson supported the publication of Stevens' paper, which was already in press when he read it. Since both Stevens and Wilson arrived at the conclusion of sex determination independently, they are both credited for their discovery. Wilson and Stevens would reference each other in their papers to support their conclusions (Citation 48). |
In 1908 Sir Archibald Garrod suggested that "inborn errors of metabolism" resulted in human diseases which in turn result from the lack of a specific enzyme (Citation 27).
Wilhelm Ludwig Johannsen was a Danish geneticist who strongly contributed to the study of modern genetics (Citation 50). Johannsen's attraction to heredity and natural selection resulted from his research in botany This led him to perform several plant experiments. Johannsen specifically experimented with bean plants in order to suggest that natural selection doesn't effect pure line species. His pure line theory suggested that any differences among the seeds must be due to chance and environment rather than natural selection. "Johannsen was the first to attribute the origin of small, continuous differences to mutation" (Citation 52). In 1909 Johannsen coined the term "gene" to describe the unit of inheritance. He stated that "The word gene is completely free of any hypothesis; it expresses only the evident fact that, in any case, many characteristics of the organism are specified in the germ cells by means of special conditions, foundations, and determiners which are present in unique, separate, and thereby independent ways - in short, precisely what we wish to call genes" (Citation 51). |  |
 | Thomas Hunt Morgan best known for his study on the fruitfly Drosophila melanogaster in 1909, as a child had shown an immense interest in natural history. Even at the age of ten, he collected birds, birds' eggs, and fossils during his life in the country. Later, during Morgan's 24-year period at Columbia University, he was attracted towards the study of cytology and the broader aspects of biological interpretation (Citation 54). In 1910 Morgan "proposed a theory of sex-linked inheritance for the first mutation discovered in white eye of a fruitfly" (Citation 27). Morgan devoloped an interest in how traits were inherited and distributed in developing organisms, leading him to wonder what caused this fly's eyes to differ from the rest. Therefore, Morgan chose to do a breeding analysis to find out more about white eyes, which would later lead to the confirmation of the chromosome theory. In doing so, Morgan would "be the first person to definitively link the inheritance of a specific trait with a particular chromosome" (Citation 55). |
Alfred Henry Sturtevant was an undergraduate at Comlumbia university when he attended one of the few lectures given by Thomas Morgan. Sturtevant was inspired by Morgan's passion for science and desired to work for him. Sturtevant worked on Drosophila melanogasterbecame one of Morgan's first students in the "Fly room" to work on (Citation 56). In 1913 Alfred Sturtevant drew a logical conclusion from Morgan's theories of crossing-over, suggesting that the information gained from these experimental crosses could be used to plot out the actual location of genes. He went on to reconstruct the first genetic map, a representation of the physical locations of several genes located on the X chromosome of Drosophila melanogaster. What is important about Sturtevant's suggestion is that it leads to a linear map. When Sturtevant analyzed Morgan's data, he found the genetic distance measured in map units of percent cross-over were additive: the distance A-B plus the distance B-C is the same as the distance A-C. It is this relation that makes recombination maps so very useful (Citation 57). |  |
 | Hermann J. Muller was fascinated by the the subject of biology before the end of his first year at Columbia College. Courses taken during college influenced him profoundly, as well as works by writers on experimental biology and physiology. Muller closely followed the research of Morgan's Drosophila, and was intimate member of the group, although he did not have opportunity for much experimental work of his own on this material until 1912 (Citation 58). In 1926, while at the University of Texas, Muller subjected male fruit flies to relatively high doses of radiation, then mated them to virgin female fruit flies. As Muller interpreted his results, radioactive particles passing through the chromosome randomly affected the molecular structure of individual genes, rendering them either inoperative or altering their chemical functions (Citation 59). |
In 1927 Muller published his paper Artificial Transmutation of the Gene. His speech later that year at the International Congress of Plant Sciences created a media sensation. As Muller himself recognized, genetic manipulation might someday be employed in industry, agriculture, and medicine. And the prospect that inherited traits might intentionally be changed or controlled --and applied to human beings--provoked widespread awe and admiration (Citation 59).
Frederick Griffith lived quietly and reclusively. The details of his life are not completely known. Importance of his work was not appreciated until well after his death. Griffith researched many kinds of microorganisms, but his most important work dealt with pneumococcus, the bacteria that can cause pneumonia (Citation 61). In 1928 Fred Griffith performed the first experiment which suggested that protein was not the genetic material. His experiment was actually fairly simple. He first injected mice with a live strain of virulent (deadly) bacteria, and not to anyone's surprise, all of those mice died. Then, he killed the virulent bacteria cells by heating them. Mice injected with these heat-killed virulent bacteria did not die. In another set of mice, Griffith injected a live non-virulent strain of bacteria, and these mice did not die. The result which Griffith expected. The surprise came when Griffith injected a group of mice with both live and non-virulent bacteria. In that group, some of the mice died. When Griffith examined those mice, he found live virulent bacteria in their blood. Griffith drew the conclusion that the genetic information in the heat-killed virulent bacteria survived the heating process and was somehow incorporated into the genetic material of the non-virulent strain to cause them to become virulent. But Griffith knew that heat denatures protein, so he suggested that the genetic material must be something else. However, his results did not specifically point to DNA as a possibility (Citation 62). |  |
Barbara McClintock had a passion for information, and in a time when a woman's career was a successful marriage, McClintock was determined to go to college. She enrolled in Cornell University, the College of Agriculture in 1918. By the time she finished her undergraduate credits, she found herself in graduate school in the new field of cytology (Citation 63). Harriet B. Creighton graduated from Wellesley in 1929 and received her Ph.D. from Cornell in 1933. She taught at Cornell and at Connecticut College, returned to Wellesley as associate professor in 1940 (Citation 64). In 1931 McClintock and Creighton demonstrated the cytological proof for crossing-over in maize. Harriet Creighton and Barbara McClintock showed by an elegantly simple experiment that exchange between genes was accompanied by exchange of cytological parts of chromosomes. The work has been acclaimed as one of the great experiments in biology. Creighton's doctoral dissertation under McClintock's membership provided the basis for the landmark paper, which was unique in merging cytological with genetic data. A companion paper by McClintock set the essential stage with data on the cytological and genetic geatures that Creighton applied. Following directly from this work, and leading to today's recognition that the genome is a graspable entity, was the knowledge that the genes could be studied as components of a linear structure, the chromosome (Citation 66). |
George Beadle was a geneticist who initially worked with the fruit fly Drosophila in the laboratory of Thomas Hunt Morgan at Columbia University. By 1935 he had developed suggestive evidence that eye color, known to be inherited, represents a series of genetically determined chemical reactions. His work over the next six years, much of it with Edward L. Tatum, a biochemist, furthered this hypothesis. But the complexity of Drosophila proved a drawback to developing experiments that would demonstrate a link between specific genes and their chemical products (Citation 67). Edward Lawrie Tatum was educated at the University of Chicago and Wisconsin, taking his A.B. degree in chemistry in 1931. Tatum's research has been concerned primarily with the biochemistry, nutrition and genetics of microorganisms and of the fruit fly, Drosophila melanogaster. During his fruitful collaboration with George Wells Beadle, he took charge of the chemical aspects of their joint work on the genetics of eye-color in Drosophila. He and Beadle decided to give up their work on Drosophila and to work instead with the fungus Neurospora crassa (Citation 68). In 1941 Beadle and Tatum Irradiated the red bread mold, Neurospora, and proved that the gene produces its effect by regulating particular enzymes. They then crossed these potential mutants with non-irradiated Neurospora. Normal products of this sexual recombination could multiply in a simple growth medium. However, Beadle and Tatum showed that some of the mutant spores would not replicate without addition of a specific amino acid—arginine. They developed four strains of arginine-dependent Neurospora—each of which, they showed, had lost use of a specific gene that ordinarily facilitates one particular enzyme necessary to the production of arginine. Beadle and Tatum's fairly simple experiment was a keystone in the development of molecular biology. From Beadle and Tatum's work arose a basic hypothesis: One gene specifies the production of one enzyme. This idea was exceptionally fruitful, but also much debated and eventually modified. Today, it is usually said, more accurately, that each gene specifies the production of a single polypeptide—that is, a protein or protein component. Thus, two or more genes may contribute to the synthesis of a particular enzyme. In addition, some products of genes are not enzymes, but structural proteins (Citation 67). |  George Beadle-(Citation 69)
 Edward Tatum-(Citation 68) |
 Avery-(Citation 70)  MacLeod-(Citation 70)  McCarty-(Citation 70) | Oswald T. Avery, an immunochemist at the Hospital of the Rockefeller Institute for Medical Research, worked for many years with pneumococcus, a bacterium that causes pneumonia. As early as 1928, he and other scientists were baffled by results of an experiment with these microbes. Mice were injected with a live but harmless form of pneumococcus and also with an inert but lethal form. Although expected to live, the mice in fact soon succumbed to infection and died. Bacteria recovered from the mice remained lethal in subsequent generations (Citation 70). Colin Munro MacLeod was a research assistant at the Rockefeller Institute for Medical Research. His research there, under the direction of Oswald Avery, focused on pneumonia and the pneumococcal infections which cause it. He examined the use of animal antiserums (liquid substances that contain proteins that guard against antigens) in the treatment of the disease. MacLeod also studied the use of sulfa drugs, synthetic substances that counteract bacteria, in treating pneumonia, as well as how pneumococci develop a resistance to sulfa drugs. He also worked on a mysterious substance then known as "C-reactive protein," which appeared in the blood of patients with acute infections (Citation 71). Maclyn McCarty majored in biochemistry under James Murray Luck, who was then launching the Annual Review of Biochemistry. McCarty presented public seminars on topics derived from articles submitted to this publication, and he graduated with a B.A. in 1933. McCarty treated children suffering from Pneumococcal pneumonia, and he was able to save a child suffering from a Streptococcal infection, then almost uniformly fatal, by the use of the newly available sulfonamide antibacterials. Both of these groups ofbacteria, Streptococcus and the Pneumococcus, would play important roles throughout the remainder of McCarty's career (Citation 72). In 1944 Avery, MacLeod, and McCarty reported that they had purified the transforming principle in Griffith's experiment and that it was DNA. As early as 1936, Avery noted that it did not seem to be a protein or carbohydrate, but a nucleic acid. Further analysis showed that it was DNA. Avery and his colleagues published a paper in the Journal of Experimental Medicine in which they set out the nature of the "transforming principle." If experimental results could be confirmed, wrote Avery, "then nucleic acids must be regarded as possessing biological specificity the chemical basis of which is as yet undetermined". Avery's paper was not initially widely read by geneticists, but it excited commentary and further research into the nature of DNA—including the relative composition of the bases that comprise it and X-ray diffraction studies of its structure (Citation 70). |
Max Delbruck has been interested in science since he was a young boy and was directed first towards astronomy. Delbruck's interest in biology was first aroused by Bohr, in connection with his speculations that the complementarity argument of quantum mechanics might have wide applications to other fields of scientific endeavor and especially in regard to the relations between physics and biology (Citation 73). In 1945 Max Delbruck organized a phage course at Cold Spring Harbor Laboratory which was taught for 26 consecutive years. This course was the training ground of the first two generations of molecular biologists (Citation 27). |  |
 | Joshua Lederberg studied at Columbia College, where he obtained his B.A. with honors in Zoology (premedical course), and from 1944 to 1946 at the College of Physicians and Surgeons of Columbia University Medical School. Here he carried out part-time research with Professor F.J. Ryan in the Department of Zoology. He was awarded hid Ph.D degree in 1948 (Citation 74). In 1946 Joshua Lederberg and Edward L. Tatum published their first paper on a type of bacterial mating called conjugation. The proof is based on the generation of daughter cells able to grow in media that cannot support growth of either of the parent cells. Their experiments showed that this type of gene exchange requires direct contact between bacteria. At the time Lederberg began studying with Tatum, scientists believed that bacteria reproduced asexually, but from the work of Beadle and Tatum, Lederberg knew that fungi reproduced sexually and he suspected that bacteria did as well (Citation 75). |
In the late 1940s Barbara McClintock developed the hypothesis of transposable elements to explain color variations in corn (Citation 27).
Erwin Chargaff was an Austro-Hungarian-born biochemist and author. He went to Yale University in the United States, where he investigated the chemical composition of lipids in Mycobacterium tuberculosis, the bacterium causing tuberculosis. He left for Europe but came back shortly after, and in 1935, started a lifelong career at Columbia University, New York (Citation 81). In 1950 Erwin Chargaff had experimentally determined certain crucial factors that led directly to the correct elucidation of its molecular structure. He demonstrated 3 rules, now known as Chargaff's Rules, which stated that in DNA: 1) The number of adenine (A) residues always equals the number of thymine (T) residues. 2) The number of guanine (G) residues always equals the number of cytosine (C) residues. 3)The number of purines (A+G) always equals the number of pyrimidines (T+C) -- this rule is an obvious consequence of rules 1 and 2. |  |
 | Rosalind Elsie Franklin excelled at science and attended one of the few girls' schools in London that taught physics and chemistry. When she was 15, she decided to become a scientist. She eventually went off to college, and after graduating, began work in Randall's lab. When Randall gave Franklin responsibility for her DNA project, no one had worked on it for months. Franklin persisted on the DNA project and J. D. Bernal called her X-ray photographs of DNA, "the most beautiful X-ray photographs of any substance ever taken." (Citation 82). Her work detailing the structures of graphitizing and non-graphitizing carbons helped form the basis for the development of carbon fibers and new heat-resistant materials, and earned her an international reputation among coal chemists (Citation 83). In 1951 Franklin obtained sharp X-ray diffraction photographs of DNA. Working with Gosling, Franklin took increasingly clear x-ray diffraction photos of DNA, and quickly discovered that there were two forms--wet and dry--which produced very different pictures. The wet form she realized was probably helical in structure, with the phosphates on the outside of the ribose chains. Her mathematical analyses of the dry form diffractions, however, did not indicate a helical structure, and she spent over a year trying to resolve the differences. By early 1953 she had concluded that both forms had two helices (Citation 83). |
Martha Cowles Chase worked as an assistant to Alfred Hershey at the Carnegie Institution of Washington in Cold Spring Harbor, New York. This was a critical period in the history of modern genetics and the beginning of an entirely new phase of research that established the science of molecular biology. Including the name of an assistant or technician on a publication, especially one that was certain to become a landmark in the history of molecular biology, was unusual during the 1960s. Thus, it is remarkable that Martha Chase's name is inextricably linked to all accounts of the path to the demonstration that DNA is the genetic material (Citation 85). Alfred Day Hershey accepted a position at the Washington University School of Medicine in the Department of Bacteriology, where he started working on bacteriophage. At the time, there weren't many people working on bacteriophage. Two other scientists who read Hershey's papers, Max Delbruck and Salvador Luria, were collaborating on experiments using bacteriophage. In 1943, Delbruck invited Hershey to Nashville to visit his lab. In 1946, working with Delbruck, Hershey discovered that phage can recombine when co-infected into a bacteria host. This led to a new area of phage genetics (Citation 88). In 1952 Alfred Hershey and Martha Chase suggested that only DNA is needed for viral replication. Using radioactive isotopes 35S to track protein and 32P to track DNA, they show that progency T2 bacteriophage isolated from lysed bacterial cells have that labeled nucleic acid. Further, most of the labeled protein doesn't enter the cells but remains attached to the bacterial cell membrane (Citation 89). |  Chase - (Citation 86)  Hershey - (Citation 87) |
 Crick - (Citation 90)  Watson - (Citation 91) | Francis H. C. Crick, a graduate student at Cavendish Laboratory in Cambridge, began working informally with the American post-doctorate James Watson. Crick had trained in physics and was engaged in X-ray studies to characterize biological molecules. Watson had studied with Salvador Luria, a pioneer in bacterial genetics. Both Crick and Watson were explicitly motivated to investigate DNA by suspicion of its fundamental significance (Citation 92). James D. Watson went to Symposium one day in May, where he met Maurice Wilkins and saw for the first time the X-ray diffraction pattern of crystalline DNA. This greatly stimulated him to change the direction of his research toward the ctructural chemistry interest in solving the DNA structure. They thought it should be possible to correctly guess its structure, given both the experimental evidence at King's College plus careful examination of the possible stereochemical configurations of polynucleotide chains. Their first serious effort, in the late fall of 1951, was unsatisfactory (Citation 91). In 1953 Watson and Crick discovered that the chemical structure of deoxyribonucleic acid (DNA) meets the unique requirements for a substance that encodes genetic information. Watson recognized how two pairs of complementary bases (adenine-thymine and guanine-cytosine) would have identical shapes if held together by hydrogen bonds. Two long chains of such base pairs would likely form a double helix—roughly, the shape of an enormously long, winding, doubled-railed staircase. The DNA molecule, comprised of long strands of such base pairs in specific and varied sequences, could embed genetic information that, if the strands were separated, could be copied. Discovery of the structure of DNA was the keystone to a half-century of research that initiated a scientific revolution. Biology acquired a molecular and biochemical basis, and research into DNA brought forth new technologies that illuminated the complex chemistry of protein synthesis and reproduction (Citation 92). |
Arthur Kornberg was first assigned to the Navy as a ship's doctor, and then as a research scientist at the National Institutes of Health (NIH) in Bethesda, Maryland, from 1942 to 1953. He obtained training in enzymology with Professor Severo Ochoa at New York University School of Medicine in 1946 and with Professor Carl Cori at Washington University School of Medicine in 1947. Upon returning to Bethesda, he organized and directed the Enzyme Section. He resigned in 1953 with the rank of Medical Director, to assume the chairmanship of the Department of Microbiology of Washington University School of Medicine in St. Louis, Missouri (Citation 93). In 1956 Kornberg crystallized DNA polymerase, the enzyme required for synthesizing DNA (Citation 94). From his early studies of the mechanisms of the enzymatic synthesis of coenzymes and inorganic pyrophosphate, he extended his interest to the biosynthesis of the nucleic acids, particularly DNA. After elucidating key steps in the pathways of pyrimidine and purine nucleotide synthesis, including the discovery of PRPP as an intermediate, he found the enzyme that assembles the building blocks into DNA, named DNA polymerase. This ubiquitous class of enzymes make genetically precise DNA and are essential in the replication, repair and rearrangements of DNA. Many other enzymes of DNA metabolism were discovered responsible for the start and elongation of DNA chains and chromosomes. These enzymes were the basis of discovery of recombinant DNA which helped ignite the biotechnology revolution (Citation 93). |  |
In 1957 Francis Crick sets out the agenda for molecular biology. Crick argued that the principal function of genes—which, as he and James Watson had suggested four years earlier, are contained in DNA—is the manufacture of proteins. Crick proposed two general principles:
The Sequence Hypothesis: The order of bases in a portion of DNA represents a code for the amino acid sequence of a specific protein. Each "word" in the code would name a specific amino acid. From the two-dimensional genetic text, written in DNA, are forged the whole diversity of uniquely shaped three-dimensional proteins.
The Central Dogma: Information is transmitted from DNA and RNA to proteins, but information cannot be transmitted from a protein to DNA.
 Meselson - (Citation 96) | Mathew Meselson had always wanted to be a chemist and had a huge lab workshop set up in his family's basement and garage. Meselson studied chemistry at the University of Chicago and then did his graduate work at the California Institute of Technology with Linus Pauling. Meselson's thesis project was to use X-rays crystallography to figure out the structure of a specific protein. In 1954 Meselson went to Woods Hole to be a teaching assistant. At Woods Hole, Meselson met Franklin Stahl --a post-doctoral fellow who was taking courses to learn some molecular biology techniques. Meselson and Stahl had a profitabe summer during which they discussed theory and possible experiments. They were especially interested in trying to devise a way to prove or disprove Watson and Crick's model of semi-conservative replication. Meselson and Stahl found themselves so in tune with each other's ideas that they agreed to work together on devising the right experiment. In 1958 Meselson and Stahl had the experimental proof for the semi-conservative replication of DNA. They did this by inventing a new technique called density gradient centrifugation, which uses centrifugal force to separate molecules based on their densities. Their "classic" paper was published and their experiment has been called "one of the most beautiful experiments in biology". Click here to watch a narrated animation of Meselson and Stahl's experiment along with it's relation to Watson and Crick's discovery. |
Francois Jacob began studying medicine at the Faculty of Paris, with the intention of becoming a surgeon. These studies were interrupted by the war. After the war, Jacob completed his medical studies and submitted his doctoral thesis in Paris in 1947. He was unable to practice surgery on account of his injuries, and worked in various fields before turning to biology. The work of Jacob has dealt mainly with the genetic mechanisms existing in bacteria and bacteriophages, and with the biochemical effects of mutations. He first studied the properties of lysogenic bacteria and demonstrated their immunity. In 1954 he began a long and fruitful collaboration with Elie Wollman, in an attempt to establish the nature of the relationships between the prophage and genetic material of the bacterium. This study led to a definition of the mechanisms of bacterial conjugation, and also enabled an analysis of the genetic apparatusof the bacterial cell. From this work there emerged a whole series of new concepts, such as the oriented process of genetic transfer from the male to the female, the circularity of the bacterial chromosome or the episome concept. The whole of this work was summarized in a book Sexuality and the Genetics of Bacteria (Citation 100). Jacques Monod obtained his Science Degree in 1931, and his doctorate in Natural Sciences in 1941. After lecturing at the Faculty of Sciences in 1934, and spending some time at the California Institute of Technology on a Rockefeller grant in 1936, Monod joined the Institut Pasteur after the liberation as Laboratory Director in Lwoff's Department. He was made Directed of the Cell Biochemistry Department in 1954, and in 1959 was appointed Professor of the Chemistry of Metabolism at the Sorbonne. In 1967 he become Professor at the College de France, and in 1971 he was appointed Director of the Institut Pasteur (Citation 101). Sydney Brenner was a South African biologist who was co-awarded the Nobel Prize in 2002 for discoveries related to apoptosis. Brenner made several seminar contributions to the field of molecular biology in the 1960s. One of his findings led Francis Crick to proposed transfer RNA. Brenner is known not only for his intellect but also his wit; for many years, he wrote a popular article for Current Biology (Citation 102). In 1960 Sydney Brenner, Francis Crick, Francois Jacob, and Jacques Monod discovered messenger RNA. They recalled research from the early 1950s with bacteriophages—viral parasites that invade bacteria. Experiments had shown that soon after bacteriophages insert their DNA into bacterial cells, traces of RNA rapidly appear. In addition, the composition of such RNA closely resembled the DNA of the invading bacteriophage. With this as context, the PaJaMo experiments suggested that another type of RNA was rapidly synthesized from DNA. Comparatively short-lived, its crucial presence had been initially overlooked. But in 1960, François Jacob and Jacques Monod named this hypothetical molecule "messenger RNA" (mRNA). Its presence was subsequently confirmed by experiment. As it was finally understood, several types of RNA represent a basic division of labor in protein synthesis. Messenger RNA (mRNA) presents information contained in DNA sequences to the ribosomes, which are structured by ribosomal RNA (rRNA). Other molecules, known as transfer RNA (tRNA), attach to specific amino acids and conduct them to the ribosomes for protein synthesis (Citation 98). |  Jacob - (Citation 98)  Monod - (Citation 98)  Brenner - (Citation 99) |
 Matthei (left) & Nirenberg (right) - (Citation 104) Leder - (Citation 108)  Khorana - (Citation 107) | Marshall Nirenberg developed an interest in biology at a very young age. In 1948 he recieved a B. Sc. degree, and in 1952, a M. Sc. degree in Zoology from the University of Florida at Gainesville. His dissertation for the Master's thesis was an ecological and taxonomic study of caddis flies (Trichoptera) (Citation 103). Johann H. Matthaei had come to the United States to complete a one-year NATO postdoctoral research fellowship. Having completed a degree in plant physiology at the University of Bonn in 1956, he arrived at the Cornell University laboratory of British-born American botanist Frederick Steward (1904-1993) hoping to conduct research on cell-free protein synthesis. Steward was not encouraging, however, and Matthaei inquired whether he could work with Nirenberg--only recently a postdoctoral fellow himself--at the National Institutes of Health (NIH). Matthaei, a technical wizard, solved many of the methodological problems that had slowed Nirenberg in his attempt to break the code (Citation 105). Philip Leder and his colleagues have contributed to the study of the genetic basis of cancer through their use of transgenic mouse models. The purpose of these studies has been to elucidate the genes that can contribute to the development of cancer, with an emphasis on identifying genes that specifically collaborate with one another to bring about cancerous growth. Leder's research indicates that the cooperative relationships between oncogenes may involve various signaling pathways within tumor cells that might contribute to the development of malignancy. Elucidating the identity of the genes and the pathways would lead to the enhanced development of therapeutic drugs for cancer (Citation 106). Har Gobind Khorana attended D.A.V. High School in Multan (now West Punjab); Ratan Lal, one of his teachers, influenced him greatly during that period. Later, he studied at the Punjab University in Lahore where he obtained an M. Sc. degree. Mahan Singh, a great teacher and accurate experimentalist, was his supervisor. In 1960 Khorana moved to the Institute for Enzyme Research at the University of Wisconsin. He became a naturalized citizen of the United States. As of the fall of 1970 Khorana has been Alfred P. Sloan Professor of Biology and Chemistry at the Massachusetts Institute of Technology (Citation 107). In 1961 Marshall Nirenberg, J. Heinrich Matthaei, Phil Leder, and Har Gobind Khorana led teams that cracked the genetic code- that triplet mRNA codons specify each of the twenty amino acids. They cracked it using using RNA homopolymer and heteropolymer experiments as well as tRNA labeling experiments (Citation 27). |
In 1961 François Jacob and Jacques Monod develop a theory of genetic regulatory mechanisms, showing how, on a molecular level, certain genes are activated and suppressed (Citation 109).
In 1967 Mary Weiss and Howard Green employ somatic cell hybridization to advance human gene mapping (Citation 110).
In 1969 Jonathan Beckwith isolates a bacterial gene (Citation 111).
Hamilton Smith studied molecular biology and genetics in his spare time, and was 31 before taking his first research post, at the University of Michigan. Smith is a co-founder of Celera Genomics, and in 1995 he led the team that accomplished the first sequencing of a bacterial genome, at the Institute for Genomic Research, where he serves as a trustee. Since 2002 he has been at the forefront of a project, funded by the U.S. Department of Energy, that seeks to create a genetically-engineered single-cell organism with the fewest genes necessary to sustain life, capable of feeding and reproducing itself (Citation 112). In 1970 alongside of Werner Arber and Daniel Nathans, Hamilton Smith discovered a new class of 'restriction enzymes' which recognize specific characteristics of nucleotides in a molecule of deoxyribonucleic acid (DNA) and cleave there, dividing the molecule at that point. This has allowed researchers to elucidate the structure and coding of DNA molecules, constructing basic genetic maps of numerous organisms, and offers the potential to correct genetic illnesses (Citation 112). |  |
 Temin - (Citation 114)  Baltimore - (Citation 115) | Howard Temin was interested in biology and during high school, he was accepted into the summer research program at Jackson Memorial Laboratory in Bar Harbor, Maine. Temin spent four summers there learning about the world of biological research. After high school, Temin went to Swarthmore College and majored in biology. In 1955, he went to graduate school at the California Institute of Technology. Although he started in biology, he became more interested in animal virology. His doctorate thesis was on work done on Rous sarcoma virus in Renato Dulbecco's laboratory. After his Ph.D. in 1959, he stayed in Dulbecco's lab for another year as a postdoctoral fellow. During this time, he developed his provirus theory, which hypothesized that RSV and other RNA viruses entered the cell and then made DNA copies of themselves before integrating into the host genome (Citation 116). Davis Baltimore studied biology and chemistry at Swarthmore College. In 1959, the summer of his third year at Swarthmore, Baltimore became one of the first undergraduate research students at Cold Spring Harbor Laboratory. He worked with George Streisinger who introduced him to the "new" field of molecular biology. As a post-doctorate, Baltimore continued to study viral systems, specifically viral RNA synthesis. In 1965, Baltimore became a research associate at the Salk Institute where he worked on poliovirus. He found that the RNA genome of poliovirus became the mRNA message once it entered the cytoplasm (Citation 117). In 1970 Howard Temin and David Baltimore independently discover reverse transcriptase, an enzyme that makes DNA from an RNA template; enzymatic isolation of DNA will become important for genetic engineering (Citation 118). |
Paul Berg knew that it is theoretically possible to create new organisms with new pathogenic abilities that could infect humans with new diseases. These diseases could be potentially deadly, since the body would not have a chance to build up any immunity against them. In 1975, Berg and others working in recombinant DNA technology recommended a set of regulations to prevent such problems, known as the "Berg Letter." These research guidelines are still in place today, although some rules have been relaxed as our control over such experimentation has increased (Citation 119). In 1972 Paul Berg assembled the first DNA molecules that combined genes from different organisms. Results of his experiments, published in 1972, represented crucial steps in the subsequent development of recombinant genetic engineering. By stepwise methods such as he devised, individual genes could be isolated and inserted into mammalian cells or into such rapidly growing organisms as bacteria. The genes themselves could then be studied, and their protein products expressed and even manufactured in quantity (Citation 120). |  |
 Boyer - (Citation 123)  Cohen - (Citation 123) | Herbert Boyer was a lineman on the varsity football team and was going to be a doctor. After high school, Boyer went to St. Vincent’s College and started with a “pre-med” curriculum. It didn’t take long for Boyer to realize that being a medical doctor was not what he wanted to do after all. By the time he graduated college in 1958 with a B.S. in biology and chemistry, Boyer had decided on a research career. He did graduate work at the University of Pennsylvania and then post-graduate work at Yale. In 1966, Boyer accepted an assistant professorship at the University of California, San Francisco. He became interested in the bacteria E. coli, specifically in the restriction enzymes that could be isolated from E. coli. At a conference in Hawaii in the early '70s, Boyer met Stanley Cohen who was working on plasmids – rings of extra chromosomal DNA. The two began a collaboration (Citation 121). Stanley N. Cohen serves as Professor of Genetics and Professor of Medicine at Stanford University School of Medicine. He was Chair of the Department of Genetics at Stanford. Dr. Cohen is a Member of Scientific and Medical Advisory Board at Charter Life Sciences, L.P. In 1973 Herbert Boyer and Stanley N. Cohen develop recombinant DNA technology, showing that genetically engineered DNA molecules may be cloned in foreign cells (Citation 123). |
Walter Gilbert explored, alongside of Benno Muller-Hill, what mechanisms a cell employs to turn genes "on" or "off." Using a radioactive tracing technique, they isolated a protein which turns off production of lactose-digesting enzymes in the bacteria E. coli. This verified the model of cellular gene expression proposed by François Jacob, André Lwoff, and Jacques Monod a few years earlier. This work led to Gilbert's sequencing of the DNA bases in and near the same genes. In 1975, at the suggestion of Andrei Mirzabekov, a Soviet scientist, Gilbert used the chemical dimethyl sulfate to fragment a strand of DNA at sites of the bases guanine and adenine. He labeled the segments with radionucleotides, cut them with restriction enzymes, which break DNA at known locations, and separated the fragments by electrophoresis. Gilbert and Allan Maxam developed a similar method for the other two DNA bases, thymine and cytosine, using the chemical hydrazine. After identifying the base sequences in the fragments, Gilbert was able to reconstruct entire DNA strand sequences thus "reading the letters" in which genetic instructions were coded (Citation 126). Frederick Sanger was awared the Corday-Morgan Medal and Prize of the Chemical Society in 1951. In 1954 he became a Fellow of the Royal Society and a Fellow of King's College, Cambridge. He is an Honarary Foreign Member of the American Academy of Arts and Sciences; Honorary Member of the American Society of Biological Chemists, Member of the Academies of Science of Argentina and Brazil, Honorary Member of the Japanese Biochemical Society, and Corresponding Member of the Association Qulmica Argentina (Citation 127). In 1977 Walter Gilbert and Frederick Sanger devised techniques for sequencing DNA. Sanger and Gilbert each took advantage of recently discovered enzymes and both methods benefited from improvements in gel electrophoresis, a method used for imaging the order of nucleotides. The methods devised by Sanger and Gilbert made it possible to read the nucleotide sequence for entire genes, which run from 1,000 to 30,000 bases long. For discovering these techniques Gilbert and Sanger received the Albert Lasker Medical Research Award in 1979, and shared the Nobel Prize in Chemistry in 1980 (Citation 128). |  Gilbert - (Citation 124)  Sanger - (Citation 125) |
 | David Botstein had made fundamental contributions to modern genetics, including the discovery of many yeast and bacterial genes and the establishment of key techniques that are commonly used today. In 1980, Botstein and thee colleagues proposed a method for mapping genes that laid the groundwork for the Human Genome Project. Botstein received his bachelor's degree from Harvard University and doctorate from the University of Michigan before teaching at the Massachusetts Institute of Technology from 1967 to 1988. He is a member of the National Academy of Sciences and the Institute of Medicine and has received numerous awards (Citation 130). In 1978 David Botstein initiated the use of restriction fragment length polymorphisms (RFLPs, pronounced "riflips") in mapping genes to indicate genetic differences among individuals (Citation 131). |
Kary B. Mullis was a chemist who received a Bachelor of Science degree in chemistry from the Georgia Institute of Technology in 1966. He earned a Ph.D. degree in biochemistry from the University of California, Berkeley, in 1972 and lectured in biochemistry there until 1973. That year, Kary became a postdoctoral fellow in pediatric cardiology at the University of Kansas Medical School, with emphasis in the areas of angiotensin and pulmonary vascular physiology. In 1977 he began two years of postdoctoral work in pharmaceutical chemistry at the University of California, San Francisco (Citation 132). In 1983 Kary Mullis conceives and helps develop polymerase chain reaction (PCR), a technology for rapidly multiplying fragments of DNA (Citation 134). |  |
 | Leroy Hood, a biologist at the California Institute of Technology and a founder of API, Hood improved the existing Sanger' method of enzymatic sequencing, which was becoming the laboratory standard. In this method, DNA to be sequenced is cut apart, and a single strand serves as a template for the synthesis of complementary strands. The nucleotides used to build these strands are randomly mixed with a radioactively labeled and modified nucleotide that terminates the synthesis. Fragments of all different lengths result. The resulting array, sent through a separation gel, reveals the order of the bases. Transferred to film, an "autoradiograph" provides a readable sequence from raw data. This data could be transferred to a computer by a human reader (Citation 135). In 1986 Leroy Hood develops the automated sequencer. In automating the process, Hood modified both the chemistry and the data-gathering processes. In the sequencing reaction itself, he sought to replace the use of radioactive labels, which were unstable, posed a health hazard, and required separate gels for each of the four DNA bases. In place of radioisotopes, Hood developed chemistry that used fluorescent dyes of different colors—one for each of the four DNA bases. This system of "color-coding" eliminated the need to run several reactions in overlapping gels. Automated sequencing was essential for the development of genomics. Together with the advances in biochemistry that included the discovery of restriction enzymes and the cascade of techniques for recombinant DNA, it made sequencing genes and whole genomes—including the human genome—feasible enterprises that could open the way to new horizons in medicine and the biological sciences (Citation 135). |
1986-1990 - Scientists began attempting to sequence the human genome.
" A coordinated effort to sequence the human genome was conceived and discussed during the mid-1980s, as it was becoming technically achievable. Biotechnology's impressive outlook gave rise to the goal of decoding, base-by-base, the entire complement of human DNA. Genetics research had made it clear to many—though not everyone agreed—that systematic analysis of the fine structure of DNA could empower biological investigation and held exceptional promise for medicine and pharmacology. In the United States, the government-funded Human Genome Project was launched in 1990" (Citation 137).
J. Craig Venter "began the race to sequence the human genome when he unexpectedly announced to a room full of genome researchers that they could just quit now, thank you, because his company would finish the job. People who like him say he never filters his thoughts and he shoots from the hip. Others have been less diplomatic, calling him an egomaniac, an idiot, and a shallow man" (Citation 138). In 1991 J. Craig Venter discovered and explained a fast and effficient way to locate genes in order to study them and use them for research using a method involving molecules called Expressed Sequence Tags (ESTs). "Shortly after the Human Genome Project got underway in 1990, J. Craig Venter, then with the National Institutes of Health (NIH), demonstrated a novel method to accelerate gene discovery. At the time, relatively few human genes had been identified and physically mapped to the genome. But molecules called Expressed Sequence Tags (ESTs) offered an efficient way to find genes and explore their functions. In addition, ESTs demonstrated the speed, accuracy and promise of automated sequencing technology" (Citation 139). |  |
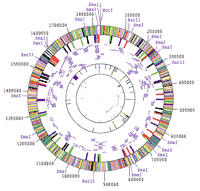 | 1995- Sequencing the genome of Haemophilus influenzae Rd. "Early proponents of the Human Genome Project recognized both the importance of innovation and the promise of sequencing the DNA of various model organisms besides human beings. By the mid-1990s, however, the principal strategies had produced complete genomes of only a few viruses. Demonstrating the value of a new strategy of "shotgun" sequencing, J. Craig Venter and colleagues published, in May 1995, the first completely sequenced genome of a self-replicating, free-living organism—the bacteria Haemophilus influenzae Rd" (Citation 140). |
1996- Yeast had its genome sequenced by many scientists. "Hundreds of scientists sequence yeast. In April 1996, some 600 scientists around the world finished sequencing the genome of baker’s yeast, an organism that carries versions of many human genes. Yeast was the third species, after two types of bacteria, to have its genome completely sequenced" (Citation 141). 1996- A microbe that can survive at the temperature of boiling water was found and brought back to a laboratory for research. "A research expedition off the coast of Mexico in 1982 discovered a microbe living near hydrothermal vents deep in the sea, where temperatures approach the boiling point of water and the pressure can crush an ordinary submarine. Using the submersible vehicle Alvin, the researchers isolated the microbe and brought it back to the laboratory" (Citation 142). | 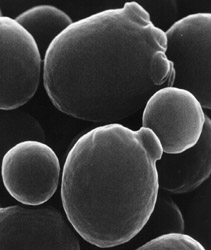 Scanning electron micrograph of S. cerevisiae.- (Citation 141) |
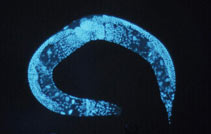 C. elegans was named for its elegant shape and movements - (Citation 143) | 1998- A microscopic worm was the first multicellular organism to have its genome sequenced. The worm, called Caenorhabditis elegans, lives in soil and grows to be a millimeter in length (Citation 143). 1999-2000- The common fruit fly, Drosophila melanogaster, was commonly used as an experimental organism for scientists to breed and crossbreed(Citation 144). |
2000- The human genome was sequenced and assembled (Citation 145). 2001- The human genome sequence is published (Citation 146). |  |
 | 2002- Researches proposed to sequence the mouse genome; claiming that the genome of a rat is closest to that present in humans (Citation 147). 2004- Baylor College of Medicine in Houston, Texas, sequenced the Brown Norway rat (Rattus norvegicus), one of the most popular strain used in research as well as a pest and pet worldwide (Citation 148). |